The new SYFOVRE Vial Kit with Injection Components (Co-Pack) is now available.
The Co-Pack should be billed using the new NDC listed below.
For GA, secondary to AMD
NAVIGATING BILLING AND CODING
Stay updated on coding information for SYFOVRE


Refer to the tools and tips below for information on billing, coding, and submitting claims for SYFOVRE.
Find the relevant codes to file your claim
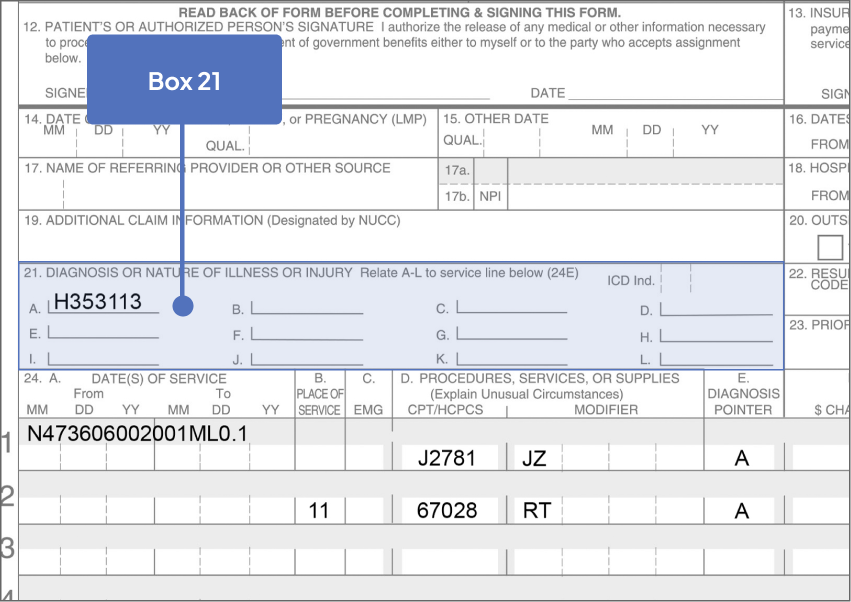
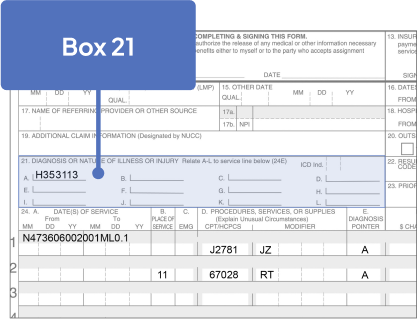
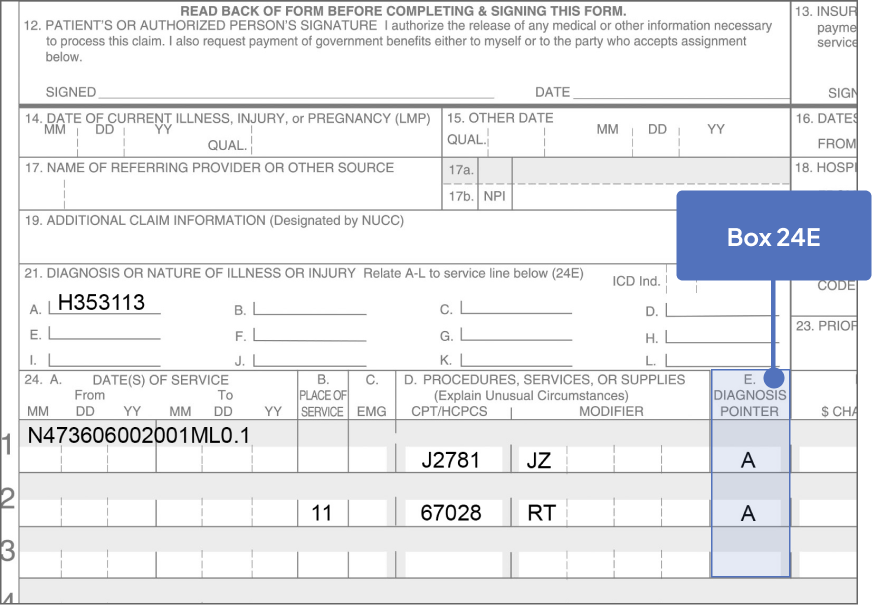
ICD-10-CM Codes for GA1
Nonexudative AMD
Advanced atrophic without subfoveal involvement
- Right eye: H35.3113
- Left eye: H35.3123
- Bilateral: H35.3133
Advanced atrophic with subfoveal involvement
- Right eye: H35.3114
- Left eye: H35.3124
- Bilateral: H35.3134
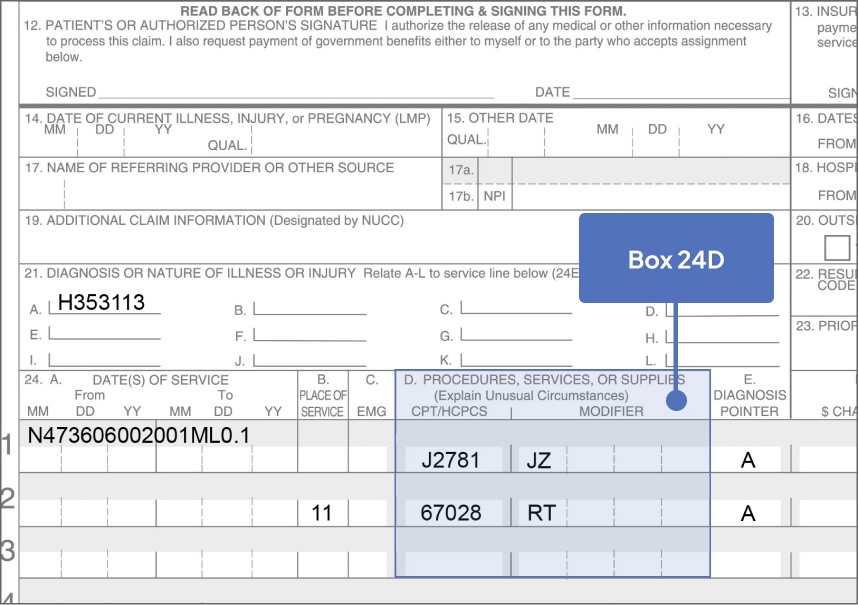
CPT Code2
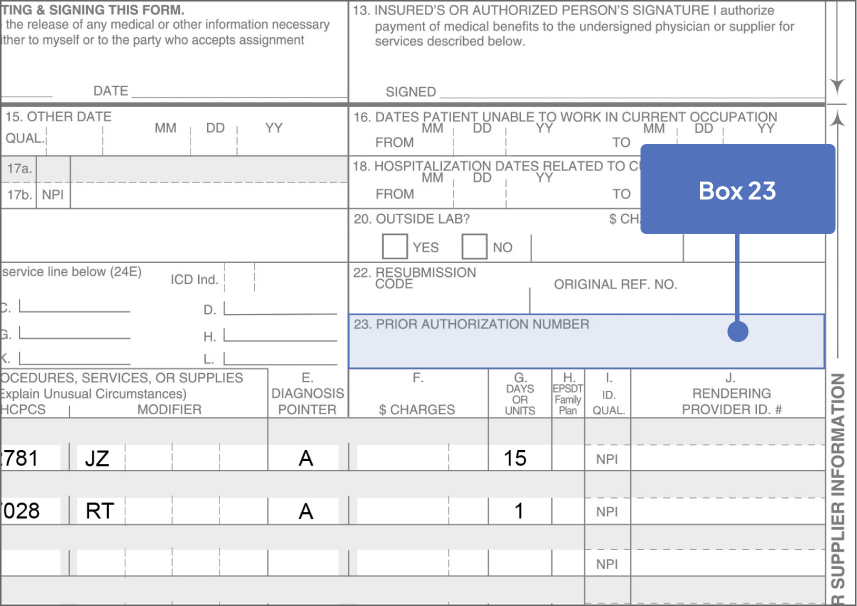
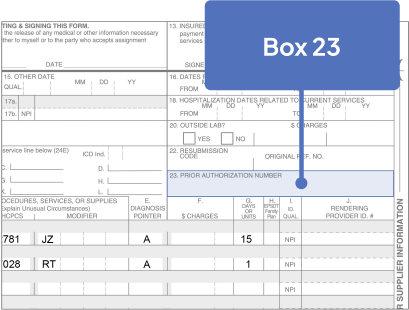
67028: Intravitreal injection of a pharmacologic agent, separate procedure
Modifiers
- Modifier
- Description
- -LT
- Left eye
- -RT
- Right eye
- -50
- Bilateral
HCPCS Code3
Permanent J-codea,b
J2781: Injection, pegcetacoplan, intravitreal, 1 mg
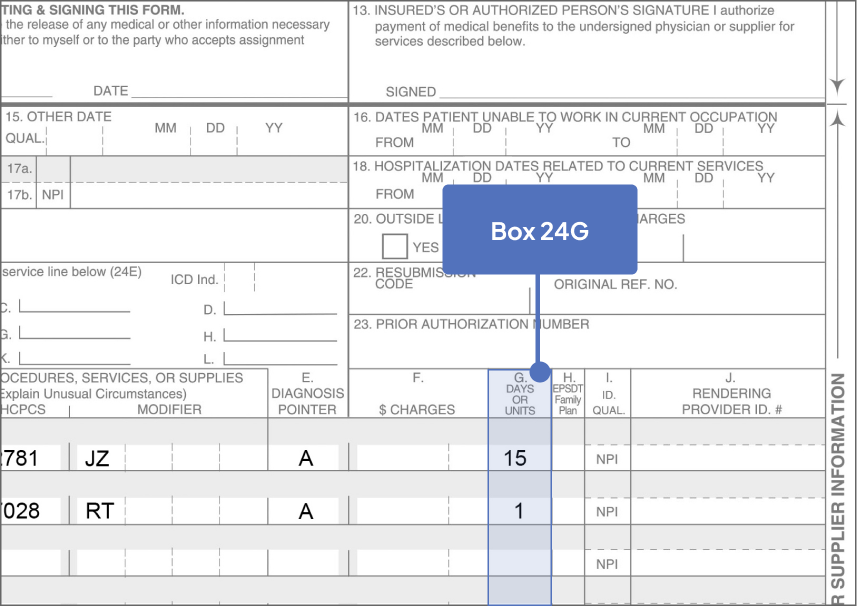
Bill 15 units for each SYFOVRE injection
The JZ modifier is required. Please check with your patient’s health plan for specific requirements
Site of care
Physician office and hospital outpatient
NDC4
10-digit NDC
- Vial only: 73606-020-01
- Vial Kit with Injection Components (Co-Pack): 73606-020-02
11-digit NDCc
- Vial only: 73606-0020-01
- Vial Kit with Injection Components (Co-Pack): 73606-0020-02
The coding information is provided for informational purposes only, is subject to change, and should not be a substitute for independent clinical judgment when selecting diagnosis and/or reimbursement codes. Codes listed above may not be exhaustive of those required by payers.
aJ2781 is a permanent, product-specific code assigned by CMS. Bill 15 units for each SYFOVRE injection.
bDose descriptor is assigned by CMS; please see full Prescribing Information for approved dosing.
cThe 11-digit NDC is derived from the 10-digit code. Many payers require the use of the 11-digit code.
Helpful tools to support access and reimbursement
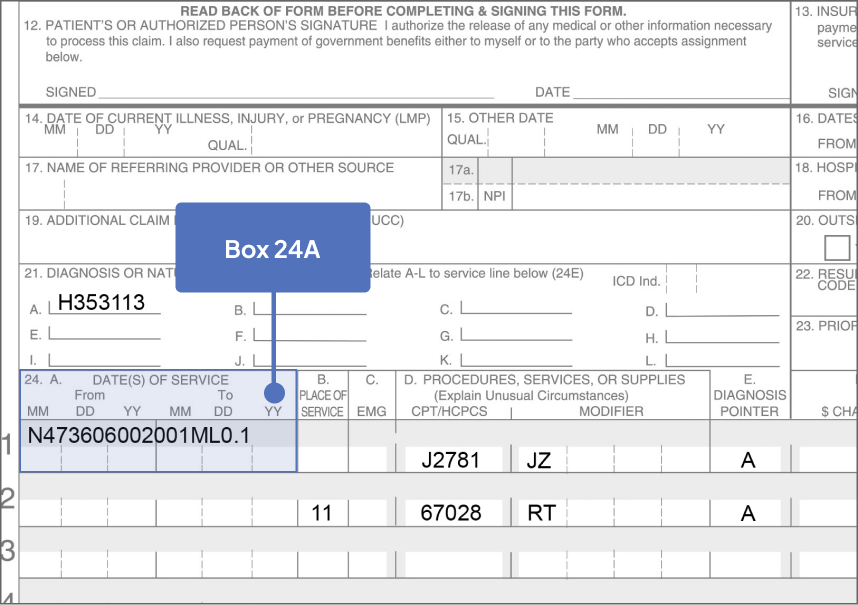
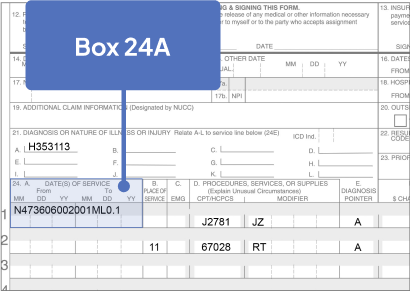
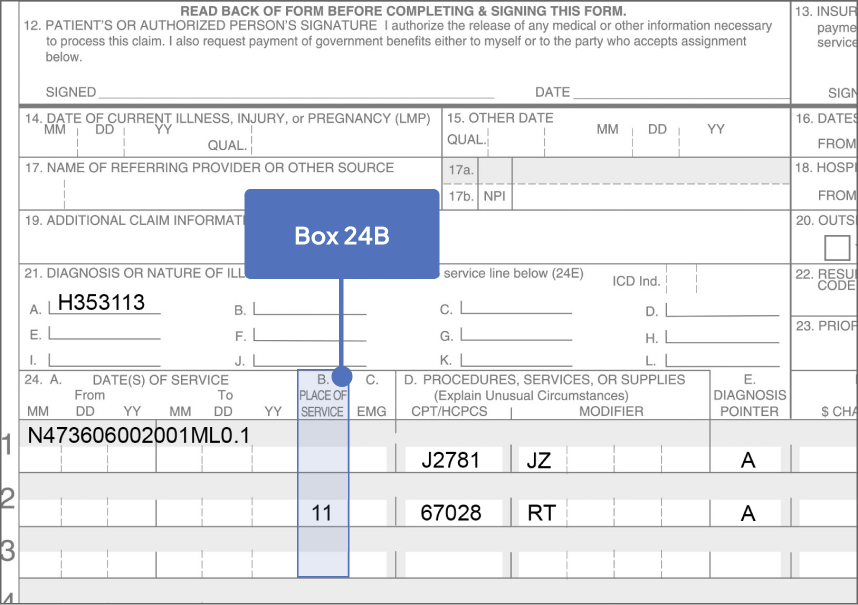
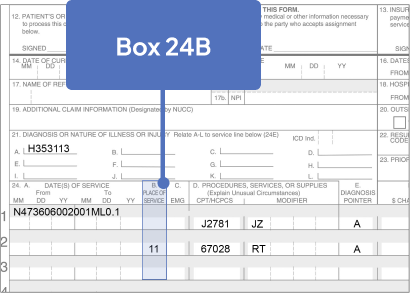
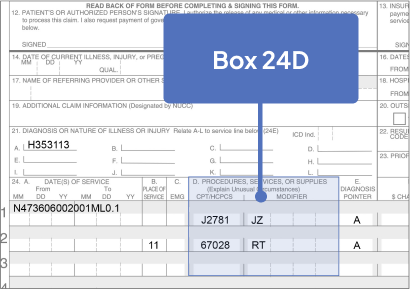
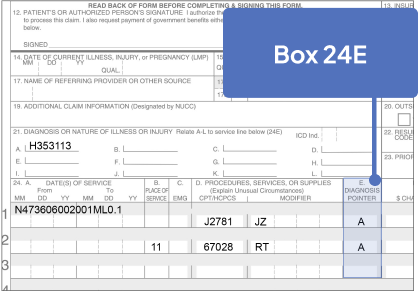
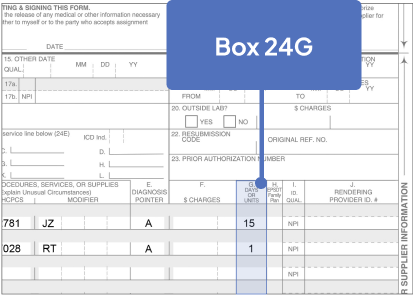
Sample CMS-1500 Claim Form: Physician Office
The sample claim form provided below is for illustrative purposes only. It is always the provider’s responsibility to determine the appropriate healthcare setting and to submit true and correct claims for the products and services rendered. Providers should contact third-party payers for specific information on their coding, coverage, payment policies, and fee schedules.
Click the boxes below to learn more
NOTE: Payers may vary in their specific billing and coding requirements. Please check with your patient’s health plan to ensure you are providing accurate and complete information.
AMD=age-related macular degeneration; CPT=Current Procedural Terminology; GA=geographic atrophy; HCPCS=Healthcare Common Procedure Coding System; ICD-10-CM=International Classification of Diseases, Tenth Revision, Clinical Modification; NDC=National Drug Code.
References: 1. Lum F, Repka MX, Vicchrilli S. How to use the ICD-10 codes for age-related macular degeneration. EyeNet Magazine. 2017;9:61-62. 2. Coding for injectable drugs. American Academy of Ophthalmology. Accessed August 16, 2024. https://www.aao.org/practice-management/coding/injectable-drugs 3. Centers for Medicare & Medicaid Services (CMS) Healthcare Common Procedure Coding System (HCPCS) application summaries and coding recommendations. Centers for Medicare & Medicaid Services. Accessed August 16, 2024. https://www.cms.gov/files/document/2023-hcpcs-application-summary-quarter-2-2023-drugs-and-biologicals.pdf 4. Syfovre. Prescribing information. Apellis Pharmaceuticals, Inc; 2023.